From Pollution to Solution
A guest post on sulfur dioxide and climate change
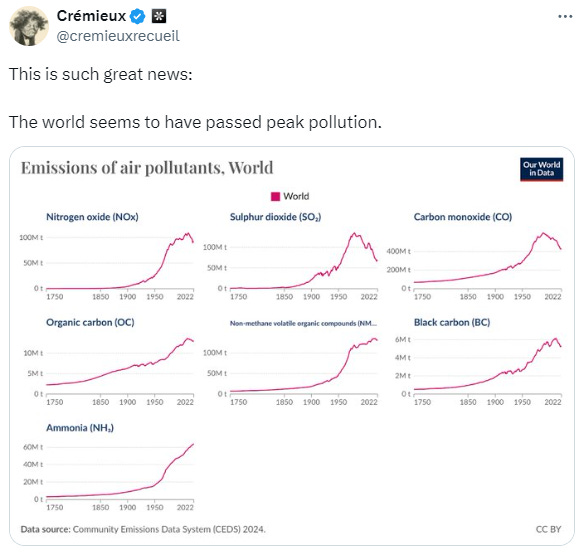
Crémieux recently tweeted that peak pollution might have been reached.
Among the jubilant voices in the comments, there were a few casting a keen eye towards the lack of sulfur dioxide (SO₂), a compound notorious for its role in acid rain. Beyond its infamous reputation, SO₂ holds a lesser-known potential as a powerful tool to combat global warming when it’s strategically deployed in the stratosphere rather than the troposphere.
This post examines the transformative role that stratospheric aerosol injection of SO₂ can play in moderating change in Earth’s climate. While many fear the corrosive effects of SO₂ based on its familiar ground-level impacts, its application in the upper atmosphere tells a different story altogether—one of cooling the Earth effectively and potentially reversing some of the most severe effects of global warming.
Chemical Reactions and the Residence Time of SO₂
Troposphere: The troposphere is the lowest layer of the Earth’s atmosphere, extending from the Earth’s surface up by about eight to fifteen kilometers (5 to 9 miles) in altitude. It’s characterized by weather phenomena, including rain, and it plays a crucial role in the formation of acid rain.
Chemical Reactions: In the troposphere, SO₂ reacts with water vapor, oxygen, and other chemicals to form sulfuric acid (H₂SO₄). The reactions are as follows:
SO₂ + OH → HOSO₂
HOSO₂ + O₂ → HO₂ + SO₃
SO₃ + H₂O → H₂SO₄
Residence Time: The residence time of SO₂—how long it lasts—in the troposphere is relatively short, typically ranging from a few days to a week, due to its removal by rain clouds.
Stratosphere: The stratosphere lies above the troposphere, extending from fifteen to fifty kilometers (9 to 31 miles) in altitude. This layer is characterized by stable atmospheric conditions and lacks weather phenomena like rain.
Chemical Reactions: In the stratosphere, SO₂ is oxidized to sulfuric acid, forming sulfate aerosols. The primary reactions include
SO₂ + OH → HOSO₂
HOSO₂ + O₂ → HO₂ + SO₃
SO₃ + H₂O → H₂SO₄
Residence Time: The residence time of SO₂ in the stratosphere is significantly longer than in the troposphere, ranging from one to three years. The absence of precipitation allows sulfate aerosols to remain suspended for extended periods, enabling their global dispersion—something kind of like a sunscreen sprayed over the Earth.
Radiative Forcing and its Climate Impact: Radiative forcing refers to the change in energy balance in the Earth’s atmosphere due to factors such as greenhouse gases and aerosols. Negative radiative forcing leads to cooling, while positive radiative forcing leads to warming.
Tropospheric SO₂
Radiative Forcing: SO₂ in the troposphere forms sulfate aerosols that reflect sunlight, causing negative radiative forcing—effecting cooling. However, these aerosols also have a short residence time and can contribute to acid rain.
Cooling Impact: The International Maritime Organization’s IMO2020 regulation significantly reduced sulfur emissions from ships. This reduction has led to a slight warming effect, as the cooling provided by the sulfate aerosols has diminished. Studies estimate that the reduction in sulfur emissions may have led to an increase in radiative forcing of about 0.1 Watts/meter². This is one of the likely reasons why 2023 has been the hottest year in recorded history. In other words, cleaner air has caused warming.
Stratospheric SO₂: A High-Altitude Solution
Radiative Forcing: When SO₂ is injected into the stratosphere, it forms sulfate aerosols that remain suspended for longer periods, effectively reflecting sunlight and causing substantial negative radiative forcing.
Cooling Impact: The eruption of Mount Pinatubo in 1991 injected approximately 15-20 million metric tons of SO₂ into the stratosphere. This resulted in a global cooling of about 0.5°C (0.9°F) over the following two years. The radiative forcing from this eruption was approximately -3 W/m² at its peak, demonstrating the significant cooling potential of stratospheric SO₂. Does anyone remember anything bad happening climate-wise in 1991? If you’re too young, ask your parents.
When moved to the stratosphere, SO₂ cools the atmosphere 1,000,000 times as effectively as CO₂ heats it. This is 25 times better leverage than tropospheric SO₂. To drive the point home, just 2.2 pounds (1 kilogram) of SO₂ in the stratosphere offsets the warming effect of ~2.2 million pounds (1 million kg) of CO₂ for a year.
SO₂ Concentrations and Acid Rain
Tropospheric Concentrations and Acid Rain
In the troposphere, SO₂ concentrations from industrial processes, coal-burning power plants, and vehicle emissions can lead to the formation of acid rain. The pH of natural rainwater is around 5.6. It’s slightly acidic due to dissolved carbon dioxide forming carbonic acid. Acid rain typically becomes a concern when the pH drops below this level due to the presence of sulfuric and nitric acids.
pH 5.0 to 5.5: Mildly acidic rain. This can begin to affect sensitive aquatic organisms and contribute to the leaching of essential nutrients from the soil.
pH 4.0 to 4.9: Moderately acidic rain. Significant harm to aquatic life, especially in soft water areas where the buffering capacity is low. Soil nutrient depletion and damage to sensitive plant species can occur.
pH below 4.0: Strongly acidic rain. Severe impacts on aquatic ecosystems, extensive soil degradation, and damage to forest ecosystems. The corrosion of buildings and monuments also becomes more pronounced.
For reference, the acidity of undiluted orange juice is a pH of 3.9.
Determining the amount of SO₂ that needs to be deployed in the stratosphere to cause mildly acidic rain involves several factors, including the dispersion and chemical conversion processes, the altitude of deployment, and regional atmospheric conditions. However, we can discuss the general principles and some estimates based on scientific studies.
Key Considerations
Dispersal and Dilution: SO₂ injected into the stratosphere disperses globally, leading to lower concentrations when it eventually descends. The dispersion reduces the localized impact compared to ground-level emissions.
Chemical Conversion: In the stratosphere, SO₂ converts to sulfuric acid aerosols. These aerosols can take one to three years to settle, during which they can spread over a wide area.
Acid Rain Formation: The eventual impact no rain pH depends on the total amount of sulfuric acid that returns to the troposphere and the volume of precipitation over which it is distributed.
Estimating Mildly Acidic Rain
Assumptions:
Mildly acidic rain has a pH of around 5 to 5.5.
The conversion efficiency of SO₂ to sulfuric acid in the stratosphere is around 70%.
The global average precipitation is approximately 1000 millimeters per year.
Calculation:
If we assume a uniform distribution of sulfuric acid aerosols, the concentration would need to be high enough to lower the pH of rain over large areas.
To make a rough estimate, we’ll consider that approximately 0.01 to 0.1 metric tons of sulfuric acid per square kilometer can significantly influence rain pH.
Given the complexity of atmospheric chemistry and the wide dispersion of aerosols, a precise estimate is challenging. However, let's outline a rough calculation:
First, we’ll determine the total area affected. To do this, we’ll assume aerosols spread uniformly over the Earth’s surface, an area of approximately 510 million square kilometers.
Second, we’ll compute the sulfuric acid required to produce mildly acidic rain. To impact our designated area, we’ll need 0.01 metric tons of sulfuric acid per square kilometer. Thus, the total required sulfuric acid is 0.01 metric tons per square kilometer * 510 million square kilometers = 5.1 million metric tons.
Third, we’ll compute the SO₂ needed to produce mildly acidic rain. Considering the conversion efficiency of 70%, the total SO₂ needed would be 5.1 million metric tons over the 70% efficiency, or approximately 7.29 million metric tons of SO₂.
Thus, we have our upper limit of 7.29 million metric tons of SO₂ that can be pumped into the atmosphere safely each year. Any more and we’ll end up with acidic rain. The next question is, does the amount bring global temperatures down meaningfully? Luckily, this exact question has been modeled extensively by scientists and featured in a report by the United Nations Environment Programme, which states:
It is estimated that continuous injection rates of 8–16 million metric tons of sulphur dioxide (SO₂) per year (approximately equivalent to the estimated injection amount of Mount Pinatubo in the single year of 1991) would reduce global mean temperature by 1°C.
The world is currently blasting through 1.5°C of warming over preindustrial levels. 7.92 million tons of SO₂ per year would buy us time. To drive the point home, let’s revisit Crémieux’s graphs and zoom into the one for SO₂.
The world tolerated 69.31 million metric tons of SO₂ in our troposphere in 2022. If we take less than 9.5% of SO₂ emissions in our troposphere and put it into the stratosphere, we would have less intense heatwaves, droughts, and wildfires, we would slow down sea level rises, and we would experience less severe storms by virtue of having a cooler Earth—all without acidic rain.
Additionally, it is possible to simultaneously cool and clean up the atmosphere by ‘redistributing’ SO₂ from the troposphere to the stratosphere. Because stratospheric SO₂ has a 25 times greater cooling effect than tropospheric SO₂, atmospheric SO₂ levels and cooling can be almost completely (96%) decoupled should we choose. The theoretical limit for redistribution while keeping or increasing cooling is less than or equal to 1/25th of human tropospheric SO₂ emissions, or 2.77 million tons of SO₂. If a regulatory body like the International Maritime Organization wanted to reduce acid rain, it would be possible to allow net atmospheric SO₂ levels to keep falling as they have since 1979 while maintaining or increasing the cooling effect we enjoy.
Long-Term Consequences and Considerations
The strategic injection of SO₂ into the stratosphere presents an innovative and promising method to address global warming. Although this area is currently unregulated and considered international territory, careful and controlled deployment of SO₂ can lead to significant ecological benefits, such as stabilizing weather patterns and potentially preserving biodiversity and water resources. Socially, it promises to mitigate adverse climate impacts on agriculture and water availability, supporting global food security and economic stability. Politically, this new frontier in climate intervention offers an opportunity to forge international collaborations that develop robust governance frameworks, ensuring that deployments are equitable and well-managed. By implementing rigorous monitoring systems and thoughtfully increasing SO₂ deployments, we can proactively manage and significantly reduce the risks associated with stratospheric aerosol injection. This adaptive and responsive approach will enable us to leverage the cooling capabilities of SO₂ while minimizing its ecological and social impacts, demonstrating a forward-thinking commitment to sustainable global climate management.
Where to Next?
The behavior of SO₂ in the troposphere has significant implications for its environmental impact. In the troposphere, SO₂ can quickly convert to sulfuric acid, leading to acid rain and associated ecological damage. In contrast, SO₂ injected into the stratosphere disperses globally and persists for a longer period of time, reducing the risk of localized acid rain but necessitating careful monitoring and research via field deployments to understand its long-term impacts. Furthermore, the radiative forcing effects of SO₂ in the stratosphere can provide significant cooling, as evidenced by historical volcanic eruptions.
The reduction of SO₂ emissions in the troposphere, such as through the IMO2020 regulation, has contributed to the warming trend observed in recent years, including making 2023 the hottest year on record. By strategically shifting a small percentage of the SO₂ emissions we currently tolerate into the stratosphere, we will leverage its cooling potential to help mitigate global warming. Although this approach carries risks, the risks of inaction are even greater. The world is already experiencing termination shock from IMO2020, and we need to reapply the "sunscreen.” But we don’t have to give anything up to do that if we deploy at a higher, safer altitude where it lasts longer and doesn’t rain out.
This one balloon releasing SO2 in the stratosphere is the equivalent of 38,636 fully-grown trees that last for a year.
About the Author
Andrew Song is a co-founder of Make Sunsets, the first company in the world to commercialize stratospheric aerosol injection as a service to cool the Earth. Make Sunsets is backed by Draper Associates, Boost VC, Pioneer Fund, and angel investors from Founders Fund and the PayPal Mafia.
You can purchase cooling credits from Make Sunsets, here.
Special thanks to Kiran Kling and Luke Iseman for feedback on this post.
Further Reading
"Chapter 30 - Sulfur dioxide: Risk assessment, environmental, and health hazard," Hazardous Gases: Risk Assessment on the Environment and Human Health, 2021. Link
"TECHNOLOGY FACTSHEET SERIES: Solar Geoengineering," Harvard University, Geoengineering Research Program. Link
"What is Acid Rain?" United States Environmental Protection Agency (EPA), 2010. Link
"Global Effects of Mount Pinatubo," NASA Earth Observatory. Link
“Analysis: How low-sulphur shipping rules are affecting global warming,” Carbon Brief, 2023. Link
"IMO2020 fuel oil sulphur limit - cleaner air, healthier planet," International Maritime Organization (IMO), 2021. Link
"One Atmosphere: An independent expert review on Solar Radiation Modification research and deployment,” United Nations Environmental Programme (UNEP), 2023. Link






Very good read. Was wondering, though, where you got the "0.1 to 0.01 metric tons per square kilometer" estimate. I am assuming this has some basis in some other calculation/research, but would appreciate a link, as that is both a wide range and not something I have a sense of how it would work (in contrast to the 70% attrition rate, which, yeah, I can see how not everything would become acid rain and 70% doesn't seem like a crazy number).
What do you think of Nephew Jonathan's suggestion to use calcium carbonate powder instead of sulfur dioxide?
https://nephewjonathan.substack.com/p/diy-geoengineering-the-whitepaper